Abstract
Introduction: While new treatments have been introduced for multiple myeloma, patients typically experience repeated relapses and progress through complex treatment pathways involving multiple lines of treatment (LOT). It remains unclear to what extent novel therapies are being utilized in treatment regimens for patients with RRMM. This study assessed treatment patterns and outcomes in patients with RRMM to better understand the current treatment landscape, unmet needs, and challenges in real-world clinical settings.
Methods: This longitudinal retrospective cohort study utilized the COTA de-identified database, which contains data from curated electronic health records of partnered US healthcare centers (index date Nov 16, 2015 to Apr 15, 2022). Study investigators identified adult patients with active RRMM who had received ≥1 prior LOT. In addition, subcohorts were created based on patients' prior exposure and refractory status: 1) prior exposure to lenalidomide (LEN-E); 2) refractory to both a proteasome inhibitor (PI) and an immunomodulatory therapy (double-class refractory [DCR]); 3) prior exposure to an anti-CD38 agent (daratumumab or isatuximab; CD38-E); 4) no prior exposure to an anti-CD38 agent (CD38-N). Demographics, clinical characteristics, and treatment patterns were analyzed descriptively. Time-to-event outcomes including (but not limited to) progression-free survival (PFS), duration of therapy (DOT), and time to next therapy (TTNT) were evaluated with Kaplan-Meier survival analysis.
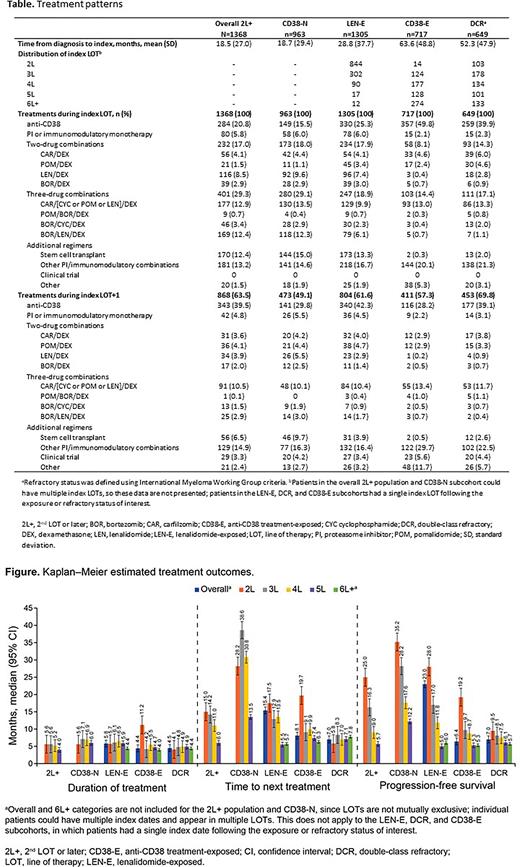
Results: In total, 1368 patients with ≥1 prior LOT were identified in the overall 2L+ population, comprising 4 overlapping subcohorts: LEN-E: 1305, DCR: 649, CD38-E: 717, CD38-N: 963; 57% were treated in an academic setting. Median age at index was 66.5 (range 27.7-96.2) yrs and 55% were male; baseline characteristics were similar across cohorts (Table). Mean time from diagnosis to index LOT was 18.5 mo (SD: 27.0). Prior to index LOT, 19.9% of patients received autologous stem cell therapy, and 25.7% of those with known ISS staging (n=997) had stage 3 disease; 43.9% had ≥1 high-risk cytogenetic features. In the overall 2L+ population at index LOT, the most common therapies were treatment regimens containing anti-CD38 agents (monotherapy or combination; 20.8%), other PI/immunomodulatory combinations (13.2%), triplets containing carfilzomib and dexamethasone (DEX) (12.9%), triplet therapy with bortezomib (BOR)/LEN/DEX (12.4%), and stem cell transplant (12.4%). In general, at later LOTs, there was a decline in use of doublets (2L: 17.0%, 3L: 13.6%, 4L: 9.6%; 5L: 11.1%) and triplets (2L: 29.3%, 3L: 15.0%, 4L: 15.4%, 5L: 11.8%), while a greater proportion of patients received anti-CD38 agents (3L: 39.5%, 4L: 42.9%, 5L: 43.9%). Treatment sequences varied greatly across cohorts. Following index LOT, 63.5% of 2L+ patients began a subsequent LOT (LOT+1). Across the subcohorts, rates of beginning LOT+1 ranged from 57.3% (CD38-E) to 69.8% (DCR) (Table 1). Given their prior exposure to anti-CD38 therapy prior to index LOT, a notably high proportion of patients in the CD38-E cohort (N=717) received an anti-CD38 agent at index LOT (49.8%), LOT+1 (28.2%), and/or LOT+2 (18.1%). Overall, median DOT was short across overall 2L+ patients and sub-cohorts, and generally remained constant across LOTs (Figure). Median PFS and TTNT varied between cohorts, with shorter durations in DCR and CD38-E cohorts. In the CD38-E patients, TTNT and PFS declined markedly across patients indexed on increasing LOTs from 2L to 6L+ (TTNT: 19.7 mo to 6.3 mo; PFS: 19.2 mo to 5.3 mo), with similar patterns seen in the LEN-E and DCR cohorts.
Conclusions: Despite development of novel therapies for MM, patients continue to receive recycled therapies at later LOTs, as demonstrated by the high rate of retreatment with prior therapies, including anti-CD38 agents. Standard of care varies by LOT and prior treatment exposure or refractory status, with no dominant regimens across cohorts. TTNT and PFS remain suboptimal across all cohorts, especially in later LOTs. These findings support the need for increased uptake of new treatments with novel mechanisms of action earlier in the patient journey, including at first relapse. This approach may provide a broader range of treatment regimens and offer additional benefits to patients with RRMM.
Funding: GSK (217353)
Disclosures
Richter:Secura Bio: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Oncopeptides: Consultancy, Honoraria; Takeda: Consultancy. Wang:GSK: Current Employment, Current equity holder in publicly-traded company. Molinari:GSK: Current Employment, Current equity holder in publicly-traded company. Gorsh:GSK: Current Employment, Current equity holder in publicly-traded company. Boytsov:GSK: Current Employment, Current equity holder in publicly-traded company. Landi:GSK: Current Employment, Current equity holder in publicly-traded company. Perera:GSK: Current Employment, Current equity holder in publicly-traded company. Paka:GSK: Current Employment, Current equity holder in publicly-traded company. Palumbo:GSK: Current Employment, Current equity holder in publicly-traded company. Duh:AstraZeneca: Research Funding; Merck: Research Funding; Shire: Research Funding; Novartis: Research Funding; Takeda Oncology: Research Funding; Blueprint Medicine: Research Funding; GSK: Research Funding; Pharmacyclics: Research Funding; Apellis Pharmaceuticals: Research Funding. Yee:Apellis Pharmaceuticals: Research Funding; RTI Health Solutions: Consultancy, Membership on an entity's Board of Directors or advisory committees. Khanal:Analysis Group: Current Employment; GSK: Research Funding. Gupta:Analysis Group: Current Employment; GSK: Research Funding. Chari:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Array Biopharma: Research Funding; Oncoceutics: Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Novartis Pharmaceuticals: Research Funding; Glaxo Smith Klein: Research Funding; Bristol Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.